Describe How to Separate Ammonia From Hydrogen and Nitrogen
The liquefied ammonia is separated and removed. Method found for pulling hydrogen from ammonia for use as clean fuel.

Haber Synthesis Of Ammonia Nh3 Nitrogen Fixation Economics Hydrogen Nitrogen Reaction Conditions Temperature Pressure Iron Catalyst Recycling Unreacted Gases Gcse Igcse Ks4 Science Chemistry O Level Revision Notes Revising
It uses a novel catalyst to crack the ammonia along with a unique membrane to separate the hydrogen from the nitrogen.

. The mixture is cooled and compressed causing the ammonia gas to condense into a liquid. Ammonia is one of the worlds most widely produced inorganic chemicals and is typically stored in tanks pressurized to about 300 psi. N H 3 333 C.
Currently the extraction of hydrogen from ammonia is carried out by two step process involving catalytic decomposition of ammonia followed by hydrogen separation to produce. The reaction mixture contains some ammonia plus a lot of unreacted hydrogen and nitrogen. If nitrogen and hydrogen were reacted at 90 atm pressure and 300 C without a catalyst some ammonia would be formed eventually.
The liquid ammonia is extracted from the mixture and the remaining hydrogen and nitrogen gases are used for another cycle of being passed over the catalyst to create more ammonia. Comparison of classical coordination compounds of ammonia and nonclassical compounds that enable bond weakening by. Ammonia in water does exist free and as the hydroxide.
The next challenge for Joe and Starfire Energy is to capture waste heat to power the cracker. Ammonia hydrogen nitrogen mixture at 400 ºC and 150 bar. So the reaction is.
Ammonia NH3 is basic and hydrazoic acid HN3 is obviously and acid. The reaction mixture contains some ammonia plus a lot of unreacted nitrogen and hydrogen. NH4-OH NH4 OH-.
The Hydrogenammonia gasses leave the evaporator and are separated by absorbing the ammonia in water. Up to 24 cash back Nitrogen - 196 ºC - 210 ºC Insoluble Hydrogen -252 ºC - 259ºC Insoluble Ammonia - 33ºC - 78 ºC Soluble Briefly describe how to separate the ammonia from hydrogen and nitrogen. The chemical formula for ammonia is NH3Chemical formula for ammonia is NH3.
System Admin Created Date. The reaction temperature of ammonia cracking into nitrogen and hydrogen is about 500C or higher. It is made up of elements nitrogen and.
The substantial difference in boiling point is about the only thing this reaction has got going for it. The mixture is cooled and compressed causing the ammonia gas to condense. H 2 2529 C.
His process reacts ammonia with sodium to produce sodium amide which then breaks down into nitrogen hydrogen and the original sodium. Northwestern University researchers have developed a highly effective environmentally friendly method for converting ammonia into hydrogen. N_2g 3H_2 g 2NH_3g Compressing the mixture Adding H_2 to the mixture Removing NH_3 from the mixture increasing the temperature of.
At first I thought about condensing the ammonia by cooling this mixture until reaching the ammonia liquefaction temperature collecting the liquid ammonia and then recycling the hydrogen and nitrogen vapors. The hydrogen rises back to the top of the system and the ammonia solution falls to the bottom. As such you dont really have to worry about the reaction with ammonium hydroxide but if you want to then.
Outlined in a recent publication in the journal Joule the new technique is a major step forward for enabling a zero-pollution hydrogen-fueled economy. Nitrogen in the reaction is obtained by separating nitrogen from the air through liquefaction and hydrogen is obtained from natural gas by steam reforming. The reaction mixture contains some ammonia plus a lot of unreacted nitrogen and hydrogen.
A mixture of gaseous nitrogen hydrogen and ammonia is placed in a sealed container and brought to equilibrium. N 2 1958 C. For example the production of an ammonia-free hydrogennitrogen gas mixture permits the.
About 46 kJmol for anhydrous ammonia 80 kJmol for aqueous. In the Haber process a pressure of 150 atm and a temperature of 450 C are used in the presence of an iron catalyst. CH 4 g H 2 O H 2 g COg According to Le Chatteleir principle the production of ammonia is favoured by high pressure and low temperature.
The device produced a non-thermal plasma via the application of voltage with a constant waveform. The formation of ammonia from nitrogen and hydrogen is mildly exothermic. The plasma in turn caused gaseous ammonia flowing axially through the plasma field to decompose into diatomic nitrogen and hydrogen NH3 e 12 N2 32 H2.
Because Ammonia is simply hydrogen and nitrogen it can be split chemically from just water H2O and air 73 nitrogen using electricity. The mixture is cooled and compressed causing the ammonia gas to. 1 2 N 2g 3 2 H 2g N H 3g The small amount of ammonia that is present at equilibrium may be condensed on a cold finger.
Which change to the system will result in an increase in the number of moles of nitrogen gas. Describe how to separate ammonia from hydrogen and nitrogen. The mixture is cooled and compressed causing the ammonia gas to condense into a liquid.
So in theory the energy cost of direct electrolytic production of ammonia from nitrogen and water is less than the cost of electrolytic production of hydrogen followed by production of ammonia from nitrogen and hydrogen. Thats about the same pressure used to store and transport propane one of the most. The device succeeded as a proof of.
A one-step cracking process will be used to convert ammonia into hydrogen and nitrogen with the hydrogen passing through the selective membrane leaving only nitrogen as the byproduct. In one ammonia molecule there is one nitrogen atom and three hydrogen atoms. It uses a novel catalyst to crack the ammonia along with a unique membrane to separate the hydrogen from the nitrogen.
D The reaction between nitrogen and hydrogen is exothermic. The hydrogen can be effectively separated by the membrane based on Pd alloy about 500C. The reaction mixture contains some ammonia plus a lot of unreacted nitrogen and hydrogen.
The idea of using ammonia as a carrier for hydrogen. The tubes were separated by a gap that varied from two to five millimeters. H-N3 H3NHN3- aka NH4N3.
Explain with reasons why the Haber process conditions are.
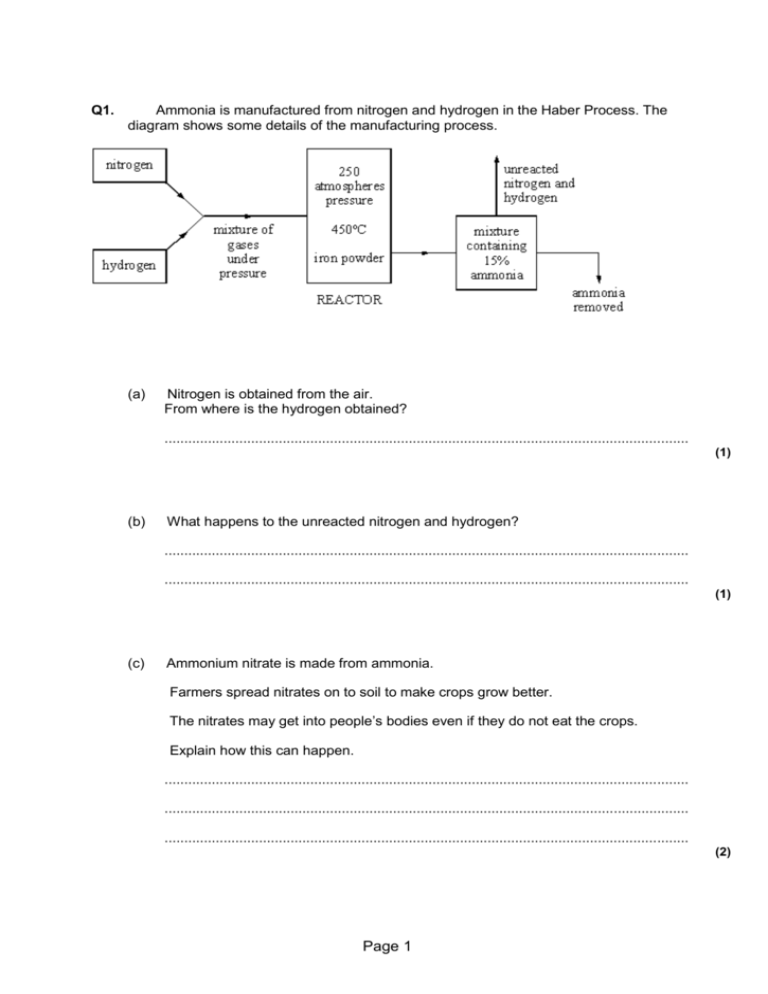
Making Ammonia And Equilibria Questions And Answers

Pdf Ammonia As Effective Hydrogen Storage A Review On Production Storage And Utilization
Synthesis Of Ammonia Process Reaction Video Lesson Transcript Study Com

Method Found For Pulling Hydrogen From Ammonia For Use As Clean Fuel
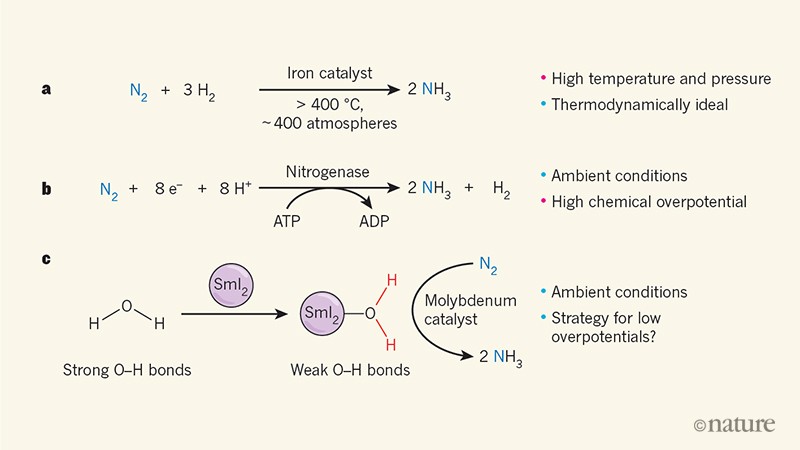
A Fresh Approach To Synthesizing Ammonia From Air And Water
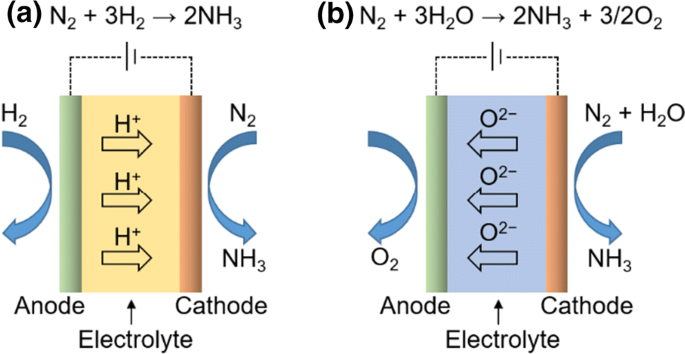
Alternative Strategies Toward Sustainable Ammonia Synthesis Springerlink

Synthesis Of Ammonia Process Reaction Video Lesson Transcript Study Com
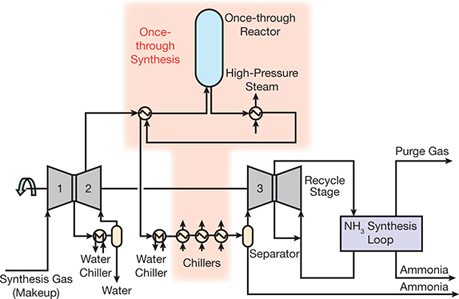
Introduction To Ammonia Production Aiche

Synthesis Of Ammonia Process Reaction Video Lesson Transcript Study Com
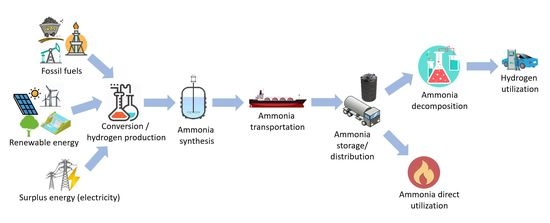
Energies Free Full Text Ammonia As Effective Hydrogen Storage A Review On Production Storage And Utilization Html

How Is Ammonia Separated From Unreacted Nitrogen And Hydrogen

Catalyst For The Carbon Free Production Of Hydrogen Gas From Ammonia
The Flow Chart Below Shows The Industrial Preparation Of Ammonia And The Process Used In The Tutorke

Synthesis Of Ammonia Process Reaction Video Lesson Transcript Study Com
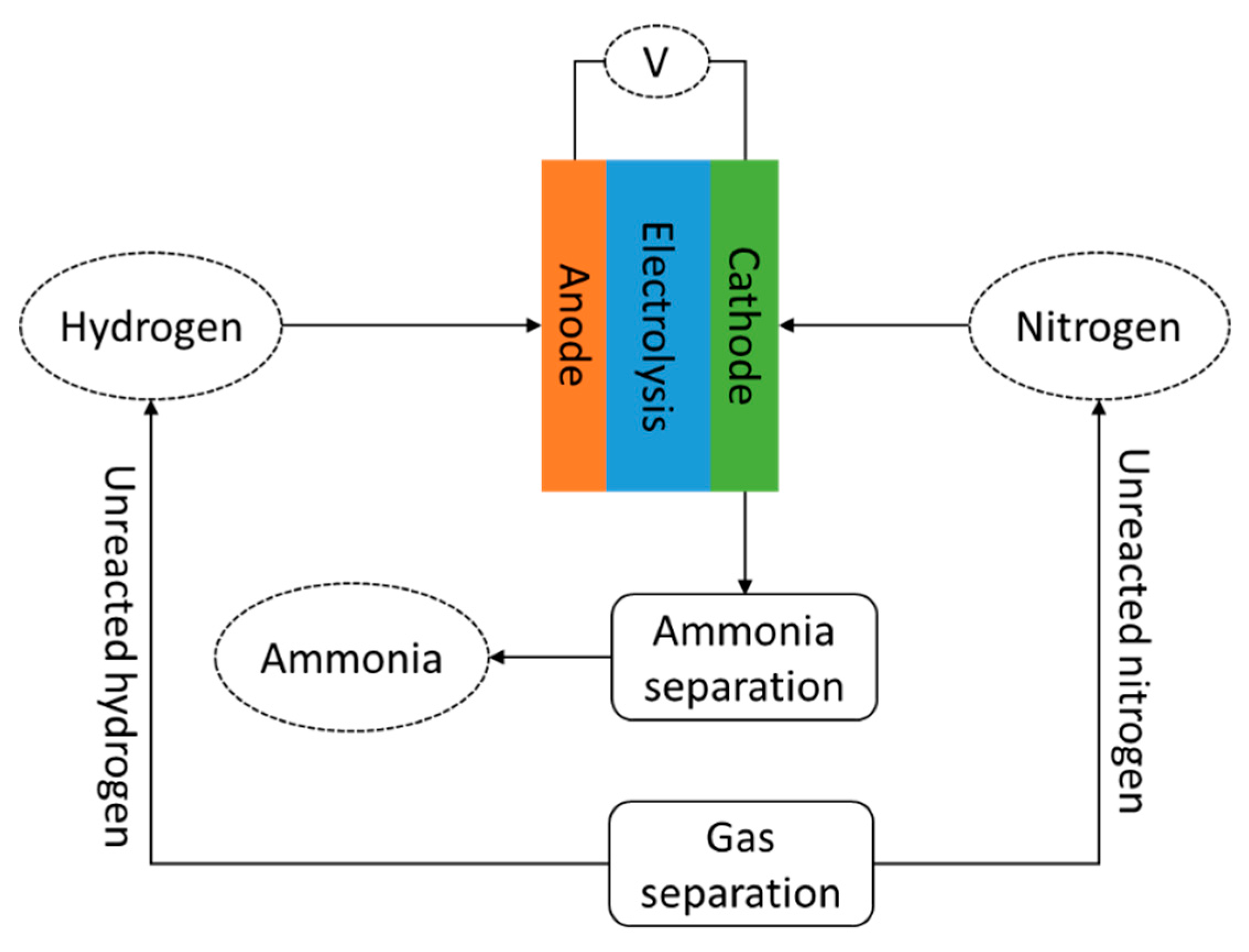
Energies Free Full Text Ammonia As Effective Hydrogen Storage A Review On Production Storage And Utilization Html
Comments
Post a Comment